问下,文献中关于CO2 势能模型里面 ε = Depth of LJ potential 的单位K 是什么意思?
manual 里面解释的ε 是 energy units
要是写pair_coeff 时,单位还用转化吗 ,我用的real单位
谢谢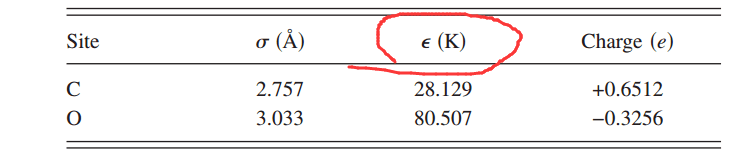
答:这里有误,正确的应该是ε/kB(K),kB是玻尔兹曼常数,1kcal/mol除以kB约503.22K
‘Nonbond Coefficients‘ (a20) [double precision] These values are listed for any non-tabular potential and they contain all of the nonbonded coefficients. These are listed with one coefficient per line. The number of nonbonded coefficients depends upon the classical_potential. The native units of the code are used (Kelvin for energy, ? for distances). If the ‘Classical Mixrule‘ is ‘Explicit‘ then the cross terms must be explicitly declared for all interactions of this atom type with any atom type that has a larger Atom Type Number. Otherwise the nonbonded coefficients are listed only for self-interaction and the mixing rule will set the cross terms. End of the section that is repeated based on the Number of Atom Type Pairs.
Energy Converter:
1 degree kelvin= 8.621738 X10-5 eV = 0.0862 meV = 0.695 cm-1
1 a.u = 27.211396 eV = 219 474.63 05 cm-1
1 Ry = 13.6057 eV
1 eV = 8065.54 cm-1
1 eV= 11,600 degrees Kelvin
1 meV = 8.065 cm-1
1 Kcal/mol= 0.0434 eV = 43.4 meV

